- Thank you received: 0
Deep-Gas, Deep Hot Biosphere Theory
- Larry Burford
-
- Offline
- Platinum Member
-

Less
More
17 years 5 months ago #18181
by Larry Burford
Replied by Larry Burford on topic Reply from Larry Burford
Shouldn't the region of vaporizing elysons be larger than the proton?
(Much larger, actually. Estimates of the relative size of atoms and their nucleii are in the range of 10^5 to one, based on things like how far apart the nucleii seem to be in typical solids and liquids.)
(Much larger, actually. Estimates of the relative size of atoms and their nucleii are in the range of 10^5 to one, based on things like how far apart the nucleii seem to be in typical solids and liquids.)
Please Log in or Create an account to join the conversation.
17 years 5 months ago #18089
by Gregg
Replied by Gregg on topic Reply from Gregg Wilson
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote"><i>Originally posted by Larry Burford</i>
<br />Shouldn't the region of vaporizing elysons be larger than the proton?
(Much larger, actually. Estimates of the relative size of atoms and their nucleii are in the range of 10^5 to one, based on things like how far apart the nucleii seem to be in typical solids and liquids.)
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
<b>Yes</b>. In fact, it would broadcast out much like a radio transmitter. But, I seriously question the assumption that a nucleus is vastly smaller than the whole atom. Consider the transformation of graphite to diamond, under tremendous pressure. Also, temperature has a <b>much greater </b>effect on the densities of liquids and solids than does pressure.
Gregg Wilson
<br />Shouldn't the region of vaporizing elysons be larger than the proton?
(Much larger, actually. Estimates of the relative size of atoms and their nucleii are in the range of 10^5 to one, based on things like how far apart the nucleii seem to be in typical solids and liquids.)
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
<b>Yes</b>. In fact, it would broadcast out much like a radio transmitter. But, I seriously question the assumption that a nucleus is vastly smaller than the whole atom. Consider the transformation of graphite to diamond, under tremendous pressure. Also, temperature has a <b>much greater </b>effect on the densities of liquids and solids than does pressure.
Gregg Wilson
Please Log in or Create an account to join the conversation.
- neilderosa
-
- Offline
- Platinum Member
-

Less
More
- Thank you received: 0
17 years 5 months ago #15089
by neilderosa
Replied by neilderosa on topic Reply from Neil DeRosa
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote">Originally posted by Larry Burford
Shouldn't the region of vaporizing elysons be larger than the proton?
(Much larger, actually. Estimates of the relative size of atoms and their nucleii are in the range of 10^5 to one, based on things like how far apart the nucleii seem to be in typical solids and liquids.)
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote">Yes. In fact, it would broadcast out much like a radio transmitter. But, I seriously question the assumption that a nucleus is vastly smaller than the whole atom. Consider the transformation of graphite to diamond, under tremendous pressure. Also, temperature has a much greater effect on the densities of liquids and solids than does pressure. [Gregg]<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
A little perspective might help here.
According to World Book Encyclopedia. Chicago: World Book, 1998: 69.
"A proton has a diameter of approximately one-millionth of a nanometer"
Convention has it that the radius of an atom is 10^-10 m
And the radius of a proton is 10^-15 m
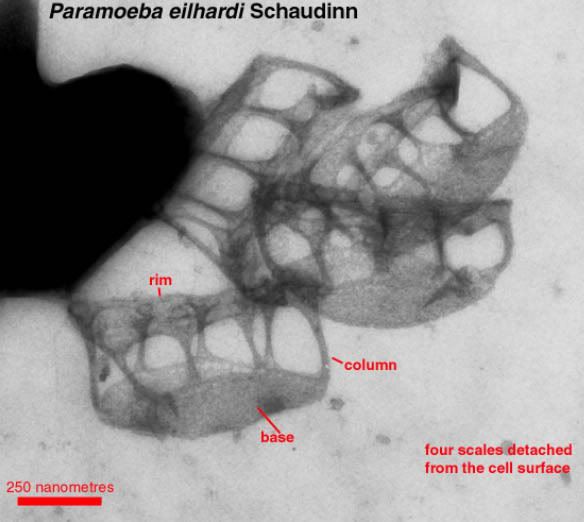
Yet this image shows cell structures down to 10^-9 m (nanometer). It’s hard to imagine that at just a little smaller scale little solar systems are floating in vast amounts of “inner space.”
Shouldn't the region of vaporizing elysons be larger than the proton?
(Much larger, actually. Estimates of the relative size of atoms and their nucleii are in the range of 10^5 to one, based on things like how far apart the nucleii seem to be in typical solids and liquids.)
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote">Yes. In fact, it would broadcast out much like a radio transmitter. But, I seriously question the assumption that a nucleus is vastly smaller than the whole atom. Consider the transformation of graphite to diamond, under tremendous pressure. Also, temperature has a much greater effect on the densities of liquids and solids than does pressure. [Gregg]<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
A little perspective might help here.
According to World Book Encyclopedia. Chicago: World Book, 1998: 69.
"A proton has a diameter of approximately one-millionth of a nanometer"
Convention has it that the radius of an atom is 10^-10 m
And the radius of a proton is 10^-15 m
Yet this image shows cell structures down to 10^-9 m (nanometer). It’s hard to imagine that at just a little smaller scale little solar systems are floating in vast amounts of “inner space.”
Please Log in or Create an account to join the conversation.
- Larry Burford
-
- Offline
- Platinum Member
-

Less
More
- Thank you received: 0
17 years 5 months ago #18341
by Larry Burford
Replied by Larry Burford on topic Reply from Larry Burford
<b>[neilderosa]"It’s hard to imagine that at just a little smaller scale little solar systems are floating in vast amounts of 'inner space.'"</b>
I have no problem imagining the billions of atoms that are depicted in this picture. But ...
===
Like others here, I do have some problems with the "little solar system" model of atoms. At the scale of the photo above an atom would be a tiny point of light (er, dark) no matter what it looks like up close. Thousands of them would be needed to make a line as long as the red bar below the label "250 nanomaters". Perhaps all you need to do is stop visualizing them as looking like solar systems, or anything else besides a point? (At least when you are standing back so far from them as in this picture. The previous posts here do seem focused on more of a close up view.)
Whether or not atoms look like solar systems, the atoms in the picture above aren't floating around. They and the parts that comprise them (protons, neutrons and electrons) are locked into rigid structures by the forces that operate among those various parts.
One of those structures labeled "column" would be dozens of atoms in diameter at the narrowest part, and thousands of atoms long from base to rim.
I have no problem imagining the billions of atoms that are depicted in this picture. But ...
===
Like others here, I do have some problems with the "little solar system" model of atoms. At the scale of the photo above an atom would be a tiny point of light (er, dark) no matter what it looks like up close. Thousands of them would be needed to make a line as long as the red bar below the label "250 nanomaters". Perhaps all you need to do is stop visualizing them as looking like solar systems, or anything else besides a point? (At least when you are standing back so far from them as in this picture. The previous posts here do seem focused on more of a close up view.)
Whether or not atoms look like solar systems, the atoms in the picture above aren't floating around. They and the parts that comprise them (protons, neutrons and electrons) are locked into rigid structures by the forces that operate among those various parts.
One of those structures labeled "column" would be dozens of atoms in diameter at the narrowest part, and thousands of atoms long from base to rim.
Please Log in or Create an account to join the conversation.
- Larry Burford
-
- Offline
- Platinum Member
-

Less
More
- Thank you received: 0
17 years 5 months ago #19802
by Larry Burford
Replied by Larry Burford on topic Reply from Larry Burford
<b>[Gregg] " ... I seriously question the assumption that a nucleus is vastly smaller than the whole atom."</b>
No one knows for sure of course. I'm pretty comfortable with the interpretation of the data from the scattering experiments that are used to "determine" these things. But not so comfortable that I'm unwilling to listen to alternative interpretations.
Of course if you are going to claim that the nucleus is only, say, one thousandth of the size of an atom, you are going to have to do lot of convincing.
No one knows for sure of course. I'm pretty comfortable with the interpretation of the data from the scattering experiments that are used to "determine" these things. But not so comfortable that I'm unwilling to listen to alternative interpretations.
Of course if you are going to claim that the nucleus is only, say, one thousandth of the size of an atom, you are going to have to do lot of convincing.
Please Log in or Create an account to join the conversation.
17 years 5 months ago #19803
by Gregg
Replied by Gregg on topic Reply from Gregg Wilson
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote"><i>Originally posted by neilderosa</i>
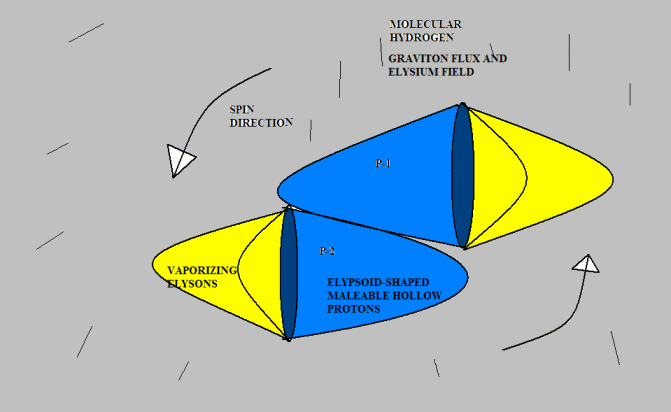
<br />Anyway, I don’t mind toying with Gregg’s idea if he doesn’t mind. Here’s a version of “molecular hydrogen.” I don’t know if this agrees with observed experimental results, but drawing from the analogy of pneumatics with which I am familiar, it seems to me that two protons would be much more likely to attach this way than tip-to-tip, because there is far more surface area to hold them together. I suggest that the protons would originally bump together tip-to-tip (because that is the most likely point of contact given their direction of travel); but they would slide down to a more stable attachment position. But I am just speculating. [Neil]
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
At least somebody is speculating!!!
The molecular hydrogen "bond" is quite weak, which is one reason I made it tip to tip (glued with liquid Elysium). But again, this issue is wide open. I don't mind speculation at all. I can't fault your option.
But I <b>do</b> mind the front door! Would have thought that post graduate work disqualified one from going trick or treating - but apparently not!
Gregg Wilson
<br />Anyway, I don’t mind toying with Gregg’s idea if he doesn’t mind. Here’s a version of “molecular hydrogen.” I don’t know if this agrees with observed experimental results, but drawing from the analogy of pneumatics with which I am familiar, it seems to me that two protons would be much more likely to attach this way than tip-to-tip, because there is far more surface area to hold them together. I suggest that the protons would originally bump together tip-to-tip (because that is the most likely point of contact given their direction of travel); but they would slide down to a more stable attachment position. But I am just speculating. [Neil]
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
At least somebody is speculating!!!
The molecular hydrogen "bond" is quite weak, which is one reason I made it tip to tip (glued with liquid Elysium). But again, this issue is wide open. I don't mind speculation at all. I can't fault your option.
But I <b>do</b> mind the front door! Would have thought that post graduate work disqualified one from going trick or treating - but apparently not!
Gregg Wilson
Please Log in or Create an account to join the conversation.
Time to create page: 0.302 seconds