- Thank you received: 0
Deep-Gas, Deep Hot Biosphere Theory
Thanks for the clear concise explanation. It appears you have the makings of an elegant new theory here (as I believe Einstein would say).
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
More likely, "Engineer gone berserk". (I did attend graduate school at Berserkeley.)
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote"><i>Originally posted by neilderosa</i>
I have more questions and comments that I’ll post as they become clear for me. But first there is one burning question; would it matter to your model if protons were “cone-shaped” or even “hat-shaped”? Perhaps made of a malleable material that could flatten out when pressure is applied in order to form a better “seal.”
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
I could very well be wrong with my four-sided pyramid. It could easily be a different shape. But the four sided pyramid seems to work quite well. HOWEVER, IT WOULD BE A GREAT ADVANCE IF OTHER PERSONS SIMPLY TOOK ON THE IDEA THAT GEOMETRY IS RELEVANT AND TRIED THEIR OWN SHAPES, ETC.
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote"><i>Originally posted by neilderosa</i>
It seems kind of hard to swallow that nature could form perfect little pyramids for us to use (figuratively speaking). One might expect something more, well, natural. But I agree with you that perfect spheres are unlikely. Neils Bohr’s “little solar system atom” seems even more of an intuitive stretch, it probably stems from ancient philosophy when spheres were thought to be the “perfect form.”
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
Here, I would say, that human preconceptions go head to head with Reality - and Reality always wins. If making protons into pyramids was done, Reality committed the "crime" only once.
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote"><i>Originally posted by neilderosa</i>
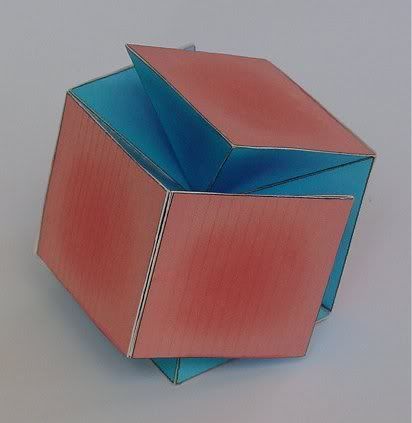
Is this something like what you mean (for coupled Helium-3)?
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
It is very close, but doesn't show the three dimensions. I need to make some paper protons, glue them together, take a photograph and post it. (don't know how to post a picture!) We moved from Bellingham, a wonderful town, down to Redmond, and I threw my "toys" out. Will redo Helium-3. (I can glue balsa part #28 to balsa part #9 but I make a lousy artist.)
Gregg Wilson
Please Log in or Create an account to join the conversation.
- neilderosa
-
- Offline
- Platinum Member
-

- Thank you received: 0
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote"> Will redo Helium-3. (I can glue balsa part #28 to balsa part #9 but I make a lousy artist.)
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
There’s more than one way to skin a cat.
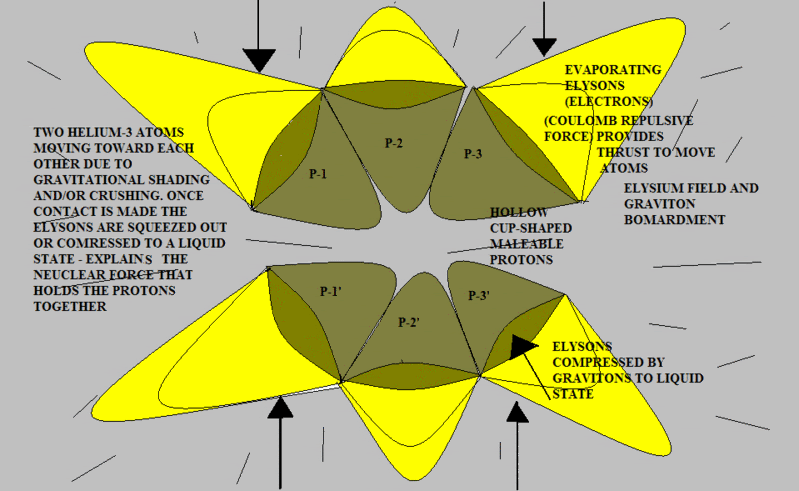
Here’s another try using slightly different shapes. Note that when the two atoms are pressed together as in the post above, (if the protons are malleable) the flattened surfaces of each “cup” will result in a shape that looks very much like a pyramid. To demonstrate this you can perform an experiment using a child’s clay by pressing a round piece together between your two thumbs and forefingers.
Please Log in or Create an account to join the conversation.
There’s more than one way to skin a cat.
Here’s another try using slightly different shapes. Note that when the two atoms are pressed together as in the post above, (if the protons are malleable) the flattened surfaces of each “cup” will result in a shape that looks very much like a pyramid. To demonstrate this you can perform an experiment using a child’s clay by pressing a round piece together between your two thumbs and forefingers.
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
Hi Neil
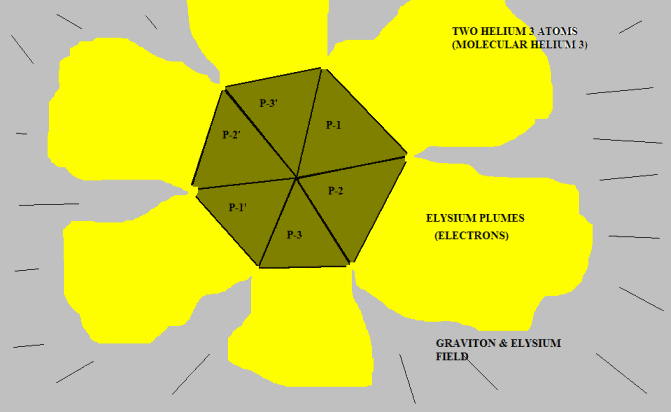
Okay. Here is two Helium-3 nuclei coupled together near absolute zero temperature:
The red surface represents the concave interior of each proton. This is the repulsive Elysium zone. The blue surfaces are simply the sides of the pyramid protons. They have been pushed together into a "fit". There is no attractive force; there is no official chemical bond.
Your option is as good as my option. What geometry is best is an open topic.
But let's analyze for a few moments. Once the two nuclei are coupled, the entire structure is now repulsive in all directions. Therefore, nothing more can be crammed into the structure. Let's consider CH4. The Carbon atom has four positions on it's nuclear surface which are not fully repulsive. Four Hydrogen atoms can be pushed into these positions. Once that is done, the CH4 molecule is fully repulsive. No further attachments can be made.
And so on. Many "fits" may not be perfect. For instance, H2O2 can easily lose an Oxygen atom because it can be better fitted into another molecule. Or, SO3 is not perfect because one more Oxygen atom can be fitted in to make SO4.
If a nucleus has a wide open area, which is not protected, and a collision at this position can cause part of the nucleus to break off, we have radioactive decay.
There is a tremendous variety of radioactive decay mechanisms just like there are a vast variety of molecular fits. This high variability almost screams "geometry involved!"
(I will not attempt to skin my cat. She has seven claws on each paw.)
Gregg Wilson
Please Log in or Create an account to join the conversation.
- neilderosa
-
- Offline
- Platinum Member
-

- Thank you received: 0
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
Your 3-D model makes it much more clear as to why there is no attractive force for a dimer of helium 3. I have an idea why near-absolute zero is required to make this bond (reduces the repusivity of surrounding elysium) but I would appreciate more accurate description.
My two attempts at drawing the model are both two-dimensional and for that reason lack descriptive power.
[Edit: I thought it would be helpfull to quote from Gregg's Oct 13th post here]
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote">Put three of them together, side to side. This is Helium-3. The common point is where one could have a "chemical bond". The bases represent the zone of "Coulomb replulsion". The "chemical bond" forms because two nuclei get in each other's way when the repulsive sides push two of them together. So, make two of the suckers. Then you "play" with them...wait, I mean "seriously maneuver them". You can demonstrate to yourself the "STRANGE, BIZZARE, UNBELIEVABLE" behavior of Helium-3 atoms:
1) Why and how they move through a vacuum to create a monoatomic layer on the poles of a magnet.
2) Why, if they collided at extreme velocity ("a million degrees"), that the only result would be an outward flux of protons (University of Wisconsin reports "140 million protons per second").
3) Why, when they are cooled down very near absolute zero, they form "dimers" which "defy gravity" and float upward and over hard surfaces.
4) Why, when Helium-3 is finally condensed under high pressure - near absolute zero temperature - it forms two, distinct, liquid phases. You can make two versions of Helium-3: one is straight and the other is a bit "kinky". Each isomer will form a dimer only with itself.
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote">Let's consider CH4. The Carbon atom has four positions on its nuclear surface which are not fully repulsive. Four Hydrogen atoms can be pushed into these positions. Once that is done, the CH4 molecule is fully repulsive. No further attachments can be made.
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
This is interesting for two reasons: It (Methane) brings us full circle to the original subject of this thread. Gold said the pressure of the earth's upper mantle was sufficient to make this molecular bond (to push the hydrogen protons into position in the carbon atom). Also, if true, one would not expect the more complex hydrocarbons (crude oil and coal) to be add-ons to methane as they seem to be.
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote">There is a tremendous variety of radioactive decay mechanisms just like there are a vast variety of molecular fits. This high variability almost screams "geometry involved!"
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
I can see how it could get very difficult to draw the larger structures using Paint, or even to make them out of paper mache. We need a talented individual to do some computer animations.
New question: TVF speaks of protons “absorbing” gravitons (e.g., in his recently posted “Structure of Matter in the Meta Model). Do you agree, or do you see gravitons as ricocheting off the surface of protons? There are more questions /comments but we’ll take them one at a time. [Neil]
Please Log in or Create an account to join the conversation.
Your 3-D model makes it much more clear as to why there is no attractive force for a dimer of helium 3. I have an idea why near-absolute zero is required to make this bond (reduces the repusivity of surrounding elysium) but I would appreciate more accurate description.
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
My opinion is that temperature should be entirely associated with Elysium. The proton's alleged asymmetry, in combination with the compressibility of Elysium, leads to movement by proton assemblies in the sense of "hotness". If we raise the temperature on Helium-3 dimers, they will simply break apart.
<blockquote id="quote"><font size="2" face="Verdana, Arial, Helvetica" id="quote">quote:<hr height="1" noshade id="quote"><i>Originally posted by neilderosa</i>
"Let's consider CH4."
This is interesting for two reasons: It (Methane) brings us full circle to the original subject of this thread. Gold said the pressure of the earth's upper mantle was sufficient to make this molecular bond (to push the hydrogen protons into position in the carbon atom). Also, if true, one would not expect the more complex hydrocarbons (crude oil and coal) to be add-ons to methane as they seem to be.
<hr noshade size="1">
Hydrocarbon formation depends on a "contest" between temperature and pressure. High temperature breaks them apart. High pressure tends to put them together.
<hr noshade size="1"><i>Originally posted by neilderosa</i>
I can see how it could get very difficult to draw the larger structures using Paint, or even to make them out of paper mache. We need a talented individual to do some computer animations.<hr noshade size="1">
My oldest son made a computer program for making these assemblies. I will try to reclaim it. I have made nuclei out of paper protons up to about carbon. It is hit or miss. The romance of cutting and gluing these suckers leaves rather quickly.
<hr noshade size="1"><i>Originally posted by neilderosa</i>
New question: TVF speaks of protons “absorbing” gravitons (e.g., in his recently posted “Structure of Matter in the Meta Model). Do you agree, or do you see gravitons as ricocheting off the surface of protons? There are more questions /comments but we’ll take them one at a time. [Neil]
<hr height="1" noshade id="quote"></blockquote id="quote"></font id="quote">
I would have to disagree. I see protons as simply contributing their shape and mass - and nothing more. If a proton can "absorb" gravitons, then it is the increase in energy that is the problem. If this increase in energy was actual, then why doesn't Pluto blaze as hot as the Sun?
I see Pluto as having very little Elysium outside of the "neutrons" and the Sun as having a vast abundance of Elysium on the outside.
I don't think that temperature is a meaningful property for protons or gravitons. Gravitons could lose momentum to elysons which will return it to the gravitons by means of electromagnetic waves, which finally "die", as their energy is transferreed back to gravitons.
However, I could very well be mistaken. (and if I learn how to edit, I will be dangerous.)
Gregg Wilson
Please Log in or Create an account to join the conversation.
- neilderosa
-
- Offline
- Platinum Member
-

- Thank you received: 0
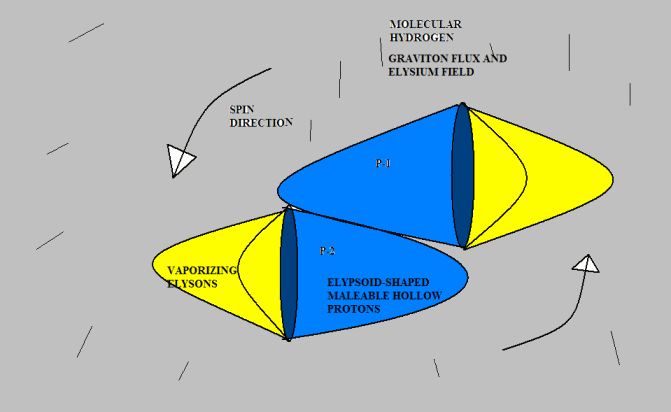
Anyway, I don’t mind toying with Gregg’s idea if he doesn’t mind. Here’s a version of “molecular hydrogen.” I don’t know if this agrees with observed experimental results, but drawing from the analogy of pneumatics with which I am familiar, it seems to me that two protons would be much more likely to attach this way than tip-to-tip, because there is far more surface area to hold them together. I suggest that the protons would originally bump together tip-to-tip (because that is the most likely point of contact given their direction of travel); but they would slide down to a more stable attachment position. But I am just speculating. [Neil]
Please Log in or Create an account to join the conversation.